EU approves new drug for locally advanced basal cell cancer
Swiss multinational pharmaceutical company, Novartis announced today that the European Commission has approved its new drug, Odomzo for the treatment of adult patients with locally advanced basal cell carcinoma (cancer) (laBCC) who are not amenable to curative surgery or radiation therapy.
The approval of Odomzo brings new hope in the form of a non-invasive option to help treat this disfiguring and potentially life-threatening disease.
Basal cell carcinoma (BCC) consists of abnormal, uncontrolled growths or lesions that arise in the skin’s basal cells, which line the deepest layer of the epidermis (the outermost layer of the skin) and accounts for more than 80% of non-melanoma skin cancers. Advanced BCC is thought to represent roughly 1-10% of all cases of BCC. Although BCC rarely becomes advanced, there have been few treatment options at this stage of the disease.
“I have seen first-hand the devastating impact advanced basal cell carcinoma can have on those living with the disease. As the lesions are usually highly visible and located predominantly on the face, they can impact patients both physically and emotionally,” said Reinhard Dummer, MD, Professor and Vice Chairman, Department of Dermatology at the University of Zurich. ”
Worldwide incidence of BCC is reportedly rising by 10% each year due to factors such as an aging population and increased ultraviolet exposure.
The EU approval of Odomzo was based on data from the Phase II randomized, double-blind, multi-center BOLT (Basal cell carcinoma Outcomes in LDE225 Trial) study in patients with laBCC not amenable to local therapy or metastatic basal cell carcinoma (mBCC).
“We are pleased to have a new treatment option for European patients living with advanced basal cell carcinoma. This milestone follows the recent approval of Odomzo in the US and is the latest example of our commitment to precision oncology and developing targeted treatments to address unmet needs,” said Bruno Strigini, President, Novartis Oncology.
About author
You might also like
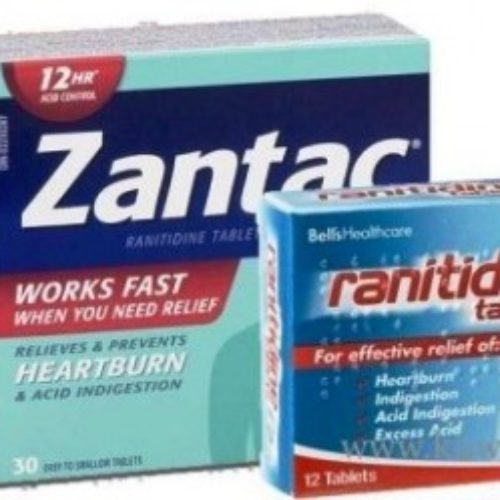
US pharmacy chains to stop sale of Zantac
Pharmacy chains in the US have moved to stop selling Zantac – a drug for heartburn – and its generic versions following the announcement by the (US) Food and Drug
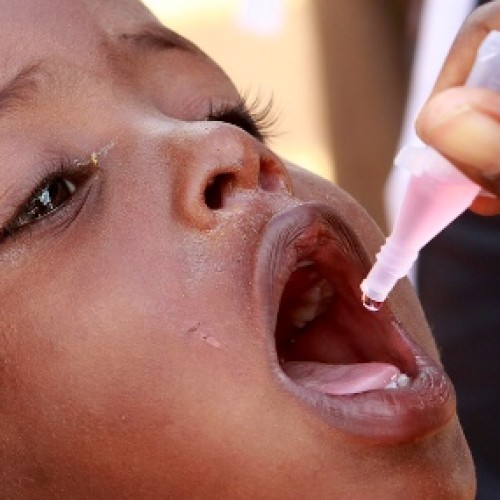
Worldwide switch to bivalent oral polio vaccine begins on Sunday
The trivalent oral polio vaccine has been instrumental in the near-eradication of the disease worldwide, but the vaccine still has some challenges that can be addressed by switching to a
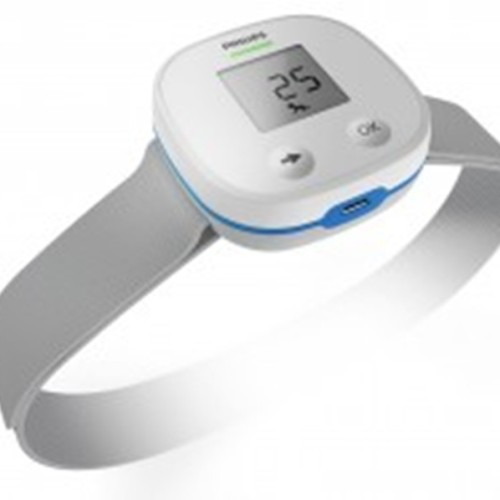
Philips introduces new diagnostic device to prevent childhood pneumonia
Royal Philips has announced the upcoming release of a Children’s Automated Respiration Monitor, aimed to help improve the diagnosis and treatment of pneumonia in low-resource countries, potentially preventing many of



0 Comments
No Comments Yet!
You can be first to comment this post!