NAFDAC alerts on recall of IV solution, children medication
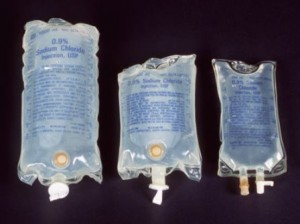
The National Agency for Food and Drug Administration and Control (NAFDAC) has announced the recall of three lots of intravenous solution by the Singaporean Health Science Authority (HSA). According to the agency, the recall was due to leakages and particulate issues which could contaminate the solution and possibly result to infection in the bloodstream, health hazards such as stroke, heart attack, damages to key organs like the kidney or liver, and even death.
Details of the affected lots are as follows:
Product code: 2B3421
Product Description: Metronidazole Injection, USP, 500mg/100mL
Lot Number: P339135
Expiration date: 31/08/2017
NDC : 0338-0553-48
Product code: 2B0043
Product Description: 0.9% Sodium Chloride Injection, USP, 100mL in mini bag plus container
Lot Number: P337857
Expiration date: 31/07/2016
NDC : 0338-0553-18
Product code: 2B7721
Product Description: Clinimix E 5/15 (5% w/Electrolyte in 15% Dextrose w/Calcium)
Lot Number: P333930
Expiration date: 31/05/2017
NDC : 0338-1123-04
NAFDAC implored importers and healthcare providers to exercise caution and discontinue the purchase, administration and use of these products. The agency also implored all outlets with the product to immediately stop circulation and submit to the nearest NAFDAC office for appropriate action.
The agency also urged healthcare providers to report all adverse events to NAFDAC PRASCOR (20543-Toll free from all networks) or via pharmacovigilance@nafdac.gov.ng
Children medication

In another development, NAFDAC has also alerted the public about the recall of some batches of children medication by the Singaporean Health Science Authority (HSA). According to the agency, the recalled products are two batches of children’s’ Guaifenesin grape liquid (100mg/5ml) and three batches of children’s’ Guaifenesin DM cherry liquid (100mg Guaifenesin and 5mg dextromethorphan HBr/5ml) contained in 4 oz. bottles with oral dosage cups in a box under multiple store brand product names.
NAFDAC reports that the recall was due to some packages containing oral dosing cups with incorrect dose markings. The products are indicated for helping loosen phlegm (mucus) and thin bronchial secretions, and in the case of the DM product to temporarily relieve: coughs due to minor throat irritations, the intensity of coughing, and the impulse to cough.
“Poor metabolizers of dextromethorphan may experience an overdose by a factor of 3, if incorrect measuring levels are used. Therefore, children who are poor metabolizers of dextromethorphan and use the product regularly over a period of several days at the mistaken dose, may develop cumulative toxicity
“Overdose of Guaifenesin DM may cause hyper excitability, rapid eye movements, changes in muscle reflexes, ataxia, dystonia, hallucinations, stupor, and coma. Other effects have included nausea, vomiting, tachycardia, irregular heartbeat, seizures, respiratory depression, and death in extreme cases,” the agency said.
Details of the affected lots are as follows:
GUAIFENESIN GRAPE LIQUID 4 OZ
Label Lot number Expiry
H.E.B 5LK0592 08/2017
CVS 5MK0340 08/2017
GUAIFENESIN DM CHERRY LIQUID 4 OZ
Label Lot number Expiry
Sunmark 5LK0528, 5LK0630 03/2017
Rite-Aid 5LK0528, 5LK0630 03/2017
Topcare 5LK0528, 5LK0630, 5LK0779 03/2017
Kroger 5LK0528, 5LK0630 03/2017
GoodSense 5LK0528 03/2017
Dollar General 5LK0630 03/2017
Care One 5LK0630 03/2017
CVS 5LK0630 03/2017
“NAFDAC implores importers and healthcare providers to exercise caution and discontinue the purchase, administration and use of these products.
“NAFDAC implores all outlets with this product to immediately withdraw them from the market.
“Healthcare providers should report all adverse events to NAFDAC PRASCOR (20543-Toll free from all networks) or via pharmacovigilance@nafdac.gov.ng,” the alert urged.
About author
You might also like
Please don’t go on strike, Buhari begs doctors
ABUJA:President Muhammadu Buhari earlier today pleaded with Nigerian resident doctors to shelve their plan to embark on a strike from April 25 and give his government a chance to address
Lagos commences COVID-19 vaccination
Gov. Sanwo-Olu, Deputy others get shots Lists frontline health workers, journalists, policemen, among first groups to be vaccinated The Lagos State government, Friday, commenced the administration of the covid-19 vaccine.
Novo Nordisk donates PPE to 12 hospitals to assist Nigeria in COVID-19 fight
L-R : Mr. Adeniji Adele (Hospital PRO); Miss Fagbenro (HOD, Health Information Management); Dr Mrs Fasanmade (Consultant Endocrinologist); Dr Oluseyi Temowo ( Chief Medical Officer, A&E); Mr Olatunbosun







0 Comments
No Comments Yet!
You can be first to comment this post!